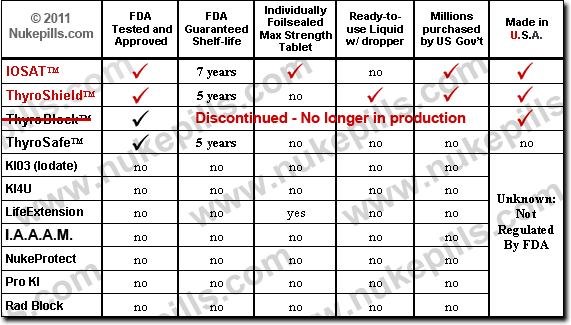
FDA Approved Brands of Potassium Iodide
Only three brands of potassium iodide (KI) have been approved by the FDA for radiation exposure – IOSAT, ThyroShield and Thyrosafe. Any other brands sold for radiation exposure are not tested and approved by the FDA and are in violation of The 1997 Federal Food, Drug and Cosmetic Act that states:
- To verify what brands of Potassium Iodide (or any drug) have FDA approval visit the FDA Orange Book. Type in Potassium Iodide, click on “OTC” and then “submit”.
Potassium Iodate (KIO3) has not been tested and approved by the FDA for anything, let alone radiation exposure. Anyone selling this drug for radiation exposure is doing so in violation of federal law (see above). The FDA has issued warning letters to sellers and manufacturers of unapproved potassium iodide and potassium iodate. Unfortunately, the FDA’s policing of this matter has waned. Many of the websites are still up and running. Some state their product is FDA approved when it isn’t!
- To verify that Potassium Iodate is not FDA approved, click on this link for the FDA Orange Book. Type in Potassium Iodate, click on “OTC” and then “submit”. None are tested and approved by the FDA.
![]() Potassium Iodate vs Potassium Iodide
Potassium Iodate vs Potassium Iodide
![]() How much Potassium Iodide should you buy?
How much Potassium Iodide should you buy?