Potassium Iodate vs Potassium Iodide
Which one should you trust for radiation protection?
Several internet-based companies have been marketing potassium iodate (KIO3) for radiation protection in place of potassium iodide (KI). Though the names are similar, the products are very different, and the US Food and Drug Administration has expressed serious concerns about the safety and effectiveness of iodate, and the fact that its manufacturers are not in conformity with FDA rules to assure safety, quality and purity of the product.
Although the FDA has been successful at removing most iodate products from store shelves, iodate can still be found on the internet and is falsely claimed to be FDA approved on the Wikipedia website. Wikipedia unwittingly allows dosage charts and other false claims concerning this unapproved drug to be posted by sellers of iodate who edit the page.
![]() View FDA warning letter to seller of Potassium Iodate
View FDA warning letter to seller of Potassium Iodate
FDA’s actions are based on the documented pharmacological advantages of Potassium Iodide (KI) compared to Potassium Iodate (KIO3) for thyroid blocking in a radiation emergency.
First, from the standpoint of safety and side effects, there is no question that potassium iodide (KI) is superior. It has been studied for over 100 years and used as a pediatric cough-cold medicine at much larger doses over this time with hardly any problems.
In fact, the FDA has concluded that for use among the general population, there are “not sufficient grounds from which to conclude, or even to suggest, a significant and quantifiable proportion of serious reactions.” (Symposium on Health Aspects of Nuclear Power Plant Incidents: Recommendations on the Use of Potassium Iodide: An FDA Update; April, 1983) The expected frequency of adverse reactions to KI in doses needed for thyroid blocking is as low as 1 in 10 million, and these are almost always mild and fully reversible.
But the same cannot be said of iodate which is far more likely to cause problems. This was noted by the World Health Organization and International Atomic Energy Agency in their working document (entitled “Guidelines for Stable Iodine Prophylaxis Following Nuclear Accidents,” dated 10/19/98) in which they compared iodide and iodate, and concluded that “Potassium iodide (KI) is the preferred alternative, since potassium iodate (KIO3) has the disadvantage of being a stronger intestinal irritant (see page 17).” In an radiation emergency where millions of people might be required to take thyroid blocking action, the problematic side effects of iodate could be significant.
Secondly, potassium iodide is also superior from an “effectiveness” standpoint. Iodide breaks down and is absorbed by the body much faster and more thoroughly compared to iodate. This was pointed out by the US National Council on Radiation Protection and in the December 15, 1978 Federal Register monograph from the US FDA which noted, “a number of factors were considered in choosing iodide (and specifically potassium iodide) over other agents such as…iodate. These factors included the degree of blocking achieved, the rapidity on onset of the blocking effect, the duration of the blocking effect, and the safety of the blocking agent.” The blocking effectiveness of potassium iodide was called “almost complete.” It was for these reasons that the FDA called iodide the “most suitable” product for radiation protection, and has never approved any iodate product for thyroid blocking use.
Besides the safety and effectiveness advantages of potassium iodide in general, the history of IOSAT Potassium Iodide in particular should not be overlooked. Iosat product received its FDA approval in 1982, and since that time nearly 60 million tablets have been manufactured and delivered. Iosat has the longest FDA approved shelf life of any radiation protective agent and meets or exceeds European requirements for purity, quality, safety and effectiveness.
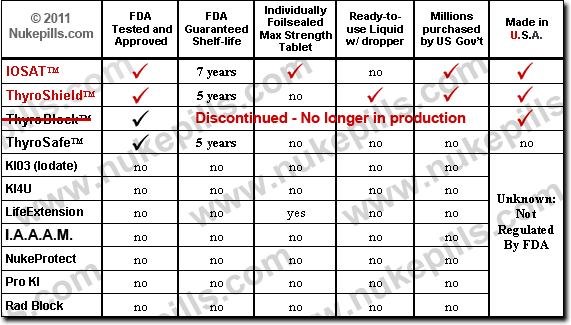
![]() What brands of Potassium Iodide are tested and verified by the FDA?
What brands of Potassium Iodide are tested and verified by the FDA?
[sc:iosatbanner]